ICH Guidelines: What They Are and Why They Matter for Medication Safety
When you take a medicine, whether it’s for fertility, blood pressure, or pain, you’re relying on a system built on ICH guidelines, international standards developed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use to ensure medicines are safe, effective, and high-quality. Also known as international harmonization standards, these rules are the invisible backbone of every approved drug you use — from the pills you buy online to the ones prescribed in clinics. Without them, one country might approve a drug based on weak data, while another rejects it. That’s not just confusing — it’s dangerous.
These guidelines cover everything from how clinical trials are designed to how side effects are reported. They’re why your blood thinner’s warning label lists specific bleeding risks, why chemotherapy disposal instructions are so strict, and why even natural supplements like turmeric or kava come with interaction warnings. The same rules that govern a new cancer drug also apply to generic versions of Zovirax or Duralast. You’ll see this in the posts below: medication safety, the practice of preventing harm from drugs through clear labeling, proper dosing, and risk monitoring, clinical trial standards, the structured methods used to test drugs in humans before approval, and pharmaceutical regulations, the legal frameworks that enforce drug quality and consistency across borders — all trace back to ICH. These aren’t abstract rules. They’re why your doctor knows to check for QT prolongation when prescribing antipsychotics, why OTC cold meds have age limits, and why your medical alert bracelet exists.
What you’ll find here isn’t a dry list of regulatory documents. It’s real-world stories — like how a supplement like turmeric spiked someone’s INR levels, or why a child’s cough medicine could cause seizures. These posts show how ICH guidelines translate into daily decisions: what’s safe to mix, what to avoid, how to spot hidden risks, and how to protect yourself. Whether you’re managing a chronic condition, navigating fertility treatment, or just trying to avoid dangerous drug interactions, these guidelines are working behind the scenes to keep you safer than you know. Below, you’ll find practical guides written by people who’ve lived through the consequences — and the solutions — of ignoring or misunderstanding them.
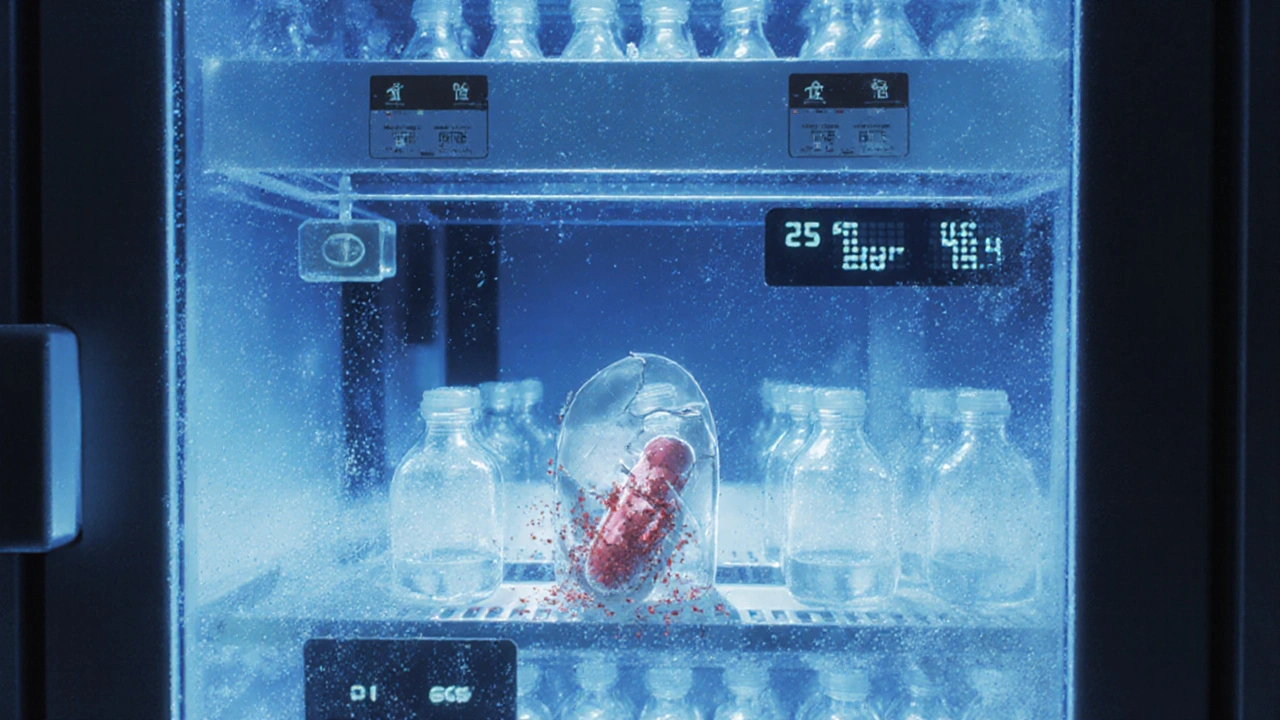
Stability Testing: Long-Term Quality Monitoring Post-Manufacture in Pharmaceuticals
Stability testing ensures pharmaceuticals remain safe and effective over time. Learn how real-time monitoring under controlled conditions prevents degradation, recalls, and patient harm-backed by ICH guidelines and real industry data.
learn more