When a pill leaves the factory, its job isn’t done. It still has to survive months-or even years-on shelves, in homes, in pharmacies, under different temperatures and humidity levels-without losing its power or turning dangerous. That’s where stability testing comes in. It’s not just paperwork or a box to check for regulators. It’s the silent guardian that ensures your medicine won’t fail you when you need it most.
Why Stability Testing Isn’t Optional
Every drug you take has a shelf life. That date on the bottle isn’t random. It’s the result of months, sometimes years, of real-world simulation inside climate-controlled chambers. The goal? To catch any changes before the product ever reaches a patient. A pill might look fine, but if its active ingredient degrades by 15%, it could stop working. Worse, it might produce toxic byproducts. In 2021, nearly 1 in 6 drug recalls in the U.S. were tied to stability failures-potency loss, unexpected chemicals, or container interactions. These aren’t theoretical risks. They’re documented failures that cost lives, money, and trust.How It Works: The Science Behind the Chambers
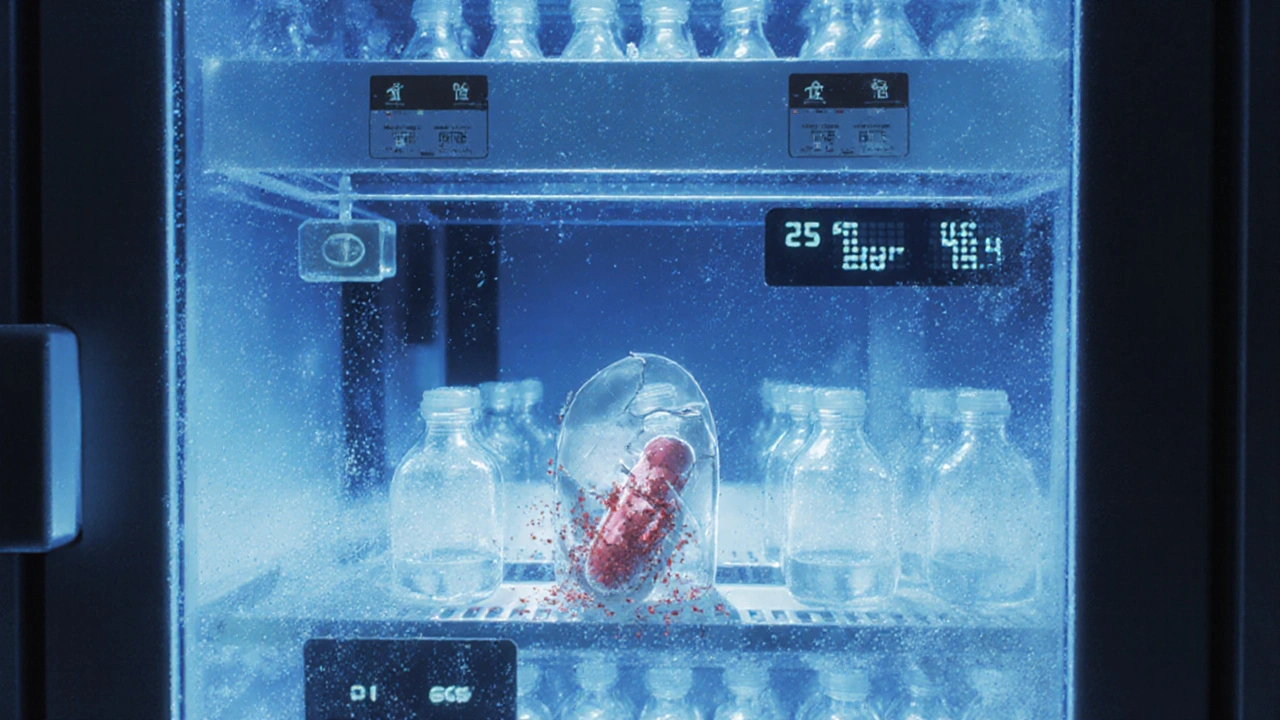
Stability testing isn’t guesswork. It follows strict, globally accepted rules laid out by the International Council for Harmonisation (ICH), especially ICH Q1A(R2). For most drugs, manufacturers place sealed products in chambers set to 25°C and 60% humidity-the standard for temperate climates like the UK or the northern U.S. Others, meant for hotter regions like Southeast Asia or India, are tested at 30°C and 65% humidity. These aren’t just room conditions; they’re exact, monitored environments with sensors logging every degree and drop of moisture. Samples are pulled at 0, 3, 6, 12, 24, and 36 months. At each point, they’re tested for:- Appearance (color, texture, clarity)
- Chemical purity (how much active ingredient remains)
- Degradation products (unwanted chemicals formed over time)
- Dissolution rate (how fast the pill breaks down in the body)
- Microbial contamination (especially for injectables and creams)
Accelerated Testing: The Shortcut That Isn’t
To speed things up, companies run accelerated tests at 40°C and 75% humidity for six months. This mimics what would happen over two to three years of real-time aging. But here’s the catch: it’s predictive, not definitive. A 2021 study in the Journal of Pharmaceutical Sciences showed that while accelerated data can flag problems early, it often misses subtle degradation patterns only visible over time. That’s why real-time testing remains the gold standard. You can’t fake 36 months of shelf life in six.
The Hidden Costs and Real-World Struggles
Running stability testing isn’t cheap. A single product study can cost between $50,000 and $150,000. For a company with 20 products, that’s over $2 million a year. Most of that goes to:- Stability chambers (each one costs $50,000-$150,000)
- Quarterly temperature mapping (around $8,500 per chamber)
- Staff trained in analytical chemistry and regulatory compliance
- Electronic data systems that meet ICH Q1D standards
Who’s Doing It Right?
Some companies are cutting waste without cutting safety. Take the approach of ICH Q12, introduced in 2018. It allows manufacturers to use post-approval changes without re-running full stability studies-for well-understood products. One mid-sized generics firm reduced sample sizes by 40% and saved $120,000 per product annually. How? By using historical data, risk assessments, and better process control. Then there’s the case of a biologic drug that looked fine on paper. Stability testing revealed a chemical reaction between the drug and its glass vial. The vial was leaching metals into the solution. The company redesigned the packaging before launch-avoiding a $500 million recall. That’s the power of testing done right.
What’s Changing in 2025?
The rules are evolving. In February 2023, ICH finalized Q13, a new guideline for stability testing in continuous manufacturing-a shift away from batch-based production. This means stability data must now be collected in real time, not just at the end of a production run. The FDA is pushing for this too. By 2025, companies using continuous manufacturing will need to prove stability at every stage, not just at the end. Another big shift? AI. Companies are starting to use machine learning to predict degradation paths based on molecular structure and environmental exposure. Early results show this can cut testing timelines by 30-40%. But regulators won’t accept predictions alone. Real data still rules. AI just helps you test smarter-not skip testing.Why This Matters to You
You might think stability testing is a corporate issue. It’s not. If a drug degrades, you might get a pill that doesn’t work. Or worse, one that makes you sick. Stability testing is the reason your blood pressure medication still lowers your pressure after a year on the shelf. It’s why your insulin hasn’t turned cloudy or lost potency. It’s why your antibiotics still kill bacteria when you need them. The system isn’t perfect. It’s slow. It’s expensive. But it’s the best tool we have. And when it works, it saves lives quietly-without headlines or fanfare.What’s Next for Stability Testing?
The future is risk-based. For simple, stable small molecules-like aspirin or metformin-there’s growing pressure to reduce testing duration. Experts argue that 36 months of data isn’t needed if the chemistry is well understood. But for complex biologics, personalized medicines, or new delivery systems (like inhalers or patches), testing will only get more intense. The WHO and FDA are already preparing for this. By 2030, stability requirements will likely expand to cover more product types, not fewer. The bottom line? Stability testing isn’t going away. It’s getting smarter. And the companies that master it-using data, technology, and science, not just compliance-will be the ones trusted to deliver the next generation of medicines.What is the main purpose of stability testing in pharmaceuticals?
The main purpose is to determine how a drug’s quality changes over time under real-world conditions like temperature and humidity. This data helps set safe expiration dates, storage instructions, and packaging requirements to ensure the medicine remains effective and safe until it’s used.
How long does stability testing usually take?
Real-time stability testing typically runs for 24 to 36 months to cover the full shelf life of a drug. Accelerated testing at higher temperatures can give early warnings in 6 months, but it doesn’t replace long-term data. Regulatory agencies require the full 24-36 months for final approval.
What are ICH Q1A(R2) guidelines?
ICH Q1A(R2) is the global standard for stability testing of new drug substances and products. It defines required testing conditions (like 25°C/60% RH), testing intervals (0, 3, 6, 12, 24, 36 months), and the types of data to collect-including chemical, physical, and microbiological properties. It’s mandatory for all new drug applications in the U.S., EU, and Japan.
Can stability testing prevent drug recalls?
Yes. Between 2020 and 2022, stability testing identified 47 drug products with dangerous degradation products before they reached the market, according to the International Pharmaceutical Aerosol Consortium. Many more recalls are avoided when early stability data triggers packaging or formulation changes.
Why do some companies outsource stability testing?
Stability testing requires expensive equipment, trained staff, and strict compliance systems. Many small biotech firms can’t afford to build their own labs. Outsourcing to CROs like SGS, Eurofins, or Charles River Laboratories is cost-effective and ensures regulatory compliance without the overhead.
Is stability testing required for generic drugs?
Yes. Generic drugs must prove they’re equivalent to the brand-name version-not just in active ingredient, but in stability too. The FDA requires full stability data as part of Abbreviated New Drug Applications (ANDAs). Without it, approval is denied.
What happens if a stability test fails?
If a sample fails (out-of-specification), the manufacturer must investigate immediately. This includes checking the test method, storage conditions, and manufacturing process. If the failure is confirmed and linked to product quality, the batch may be rejected, the shelf life shortened, or the product withdrawn. Repeated failures can delay regulatory approval or trigger FDA inspections.
How does stability testing affect drug pricing?
Stability testing adds significant cost to drug development-often $500,000 to $2 million per company annually. These costs are factored into pricing, especially for complex drugs like biologics. However, skipping or cutting corners on testing leads to recalls, delays, and lost sales, which cost far more in the long run.
Are there alternatives to traditional stability testing?
Accelerated testing and predictive modeling using AI are becoming supplements, but not replacements. Real-time testing under real conditions remains the only accepted method for regulatory approval. Newer approaches like Quality by Design (QbD) help reduce testing burden by building stability into the product from the start, but they still require validation with real data.
What role does packaging play in stability testing?
Packaging is a critical part of stability testing. A pill might be stable on its own, but if the blister pack lets in moisture or the vial leaches chemicals, the product fails. Tests include exposure to light, humidity, and even oxygen permeability. Many failures are traced back to packaging, not the drug itself.
Richard Wöhrl
November 22, 2025 AT 17:57Just read this and had to pause-this is the invisible infrastructure keeping millions alive. I’ve worked in pharma QA for 12 years, and stability testing is the quiet hero no one talks about until something goes wrong. That 1 in 6 recall stat? Real. I’ve seen batches pulled because a desiccant failed in humid climates. No drama, no press release-just a phone call and a lot of paperwork. But that’s the job.
Pramod Kumar
November 23, 2025 AT 22:17Bro, in India we get pills that feel like they’ve been through a monsoon and then a microwave. I’ve seen tablets crumble in my pocket just from sweat. But this? This is why some brands actually work here. The ones that test properly? They cost more, yeah-but they don’t kill you. I’d pay double for a pill that doesn’t turn into chalk after 3 months in my bathroom.
Brandy Walley
November 24, 2025 AT 11:58John Mackaill
November 25, 2025 AT 21:39Brandy’s got a point in spirit-but the data doesn’t lie. I’ve seen patients on anticoagulants whose INR spiked because their warfarin degraded in a hot car. No one knew. No one checked. The pill looked fine. That’s the danger. It’s not about fear. It’s about the fact that chemistry doesn’t care how you feel about it. If the molecule breaks down, it breaks down. And your body doesn’t ask for permission before it reacts.
Katy Bell
November 26, 2025 AT 04:36I used to work in a pharmacy and saw so many elderly patients taking meds past their expiry date because they couldn’t afford new ones. It broke my heart. But then I remembered-some of those pills were still fine. The expiry date isn’t a cliff. It’s a safety margin. And stability testing? It’s the reason that margin exists. Not perfect. But better than nothing.
shreyas yashas
November 27, 2025 AT 14:52Real talk: I’m from a small town in Karnataka. Our local clinic gets generic meds shipped from all over. Some packages are cracked, some are old. But the ones that passed stability testing? They work. I’ve seen diabetics who switched from a cheap brand to one with proper testing-sugar levels stabilized. No magic. Just science. And yeah, it’s expensive. But so is hospitalization.
Ragini Sharma
November 27, 2025 AT 16:19so like… if ai can predict degradation why are we still paying for those giant friggin’ chambers? i mean come on. we got neural nets that can guess your mood from a selfie. can’t it guess if a pill’s gonna turn into poison? also who’s paying for all this? my insurance? lol
Suresh Ramaiyan
November 28, 2025 AT 10:42There’s a beautiful irony here. We spend millions to ensure a tiny pill doesn’t change over time-yet we live in a world that’s constantly shifting. Climate change means storage conditions are no longer predictable. A drug tested in 25°C might now sit in a 35°C apartment. We’re testing for yesterday’s world. The real challenge isn’t the lab-it’s the fact that the world outside the lab is no longer stable. And we’re not adapting fast enough.
Vivian C Martinez
November 29, 2025 AT 14:08Thank you for writing this with such clarity. I’ve been trying to explain this to my cousin who thinks all drugs are the same. She doesn’t realize that the difference between a $5 generic and a $50 brand isn’t just marketing-it’s the 36 months of controlled testing, the validated HPLC methods, the humidity logs, the investigators digging into OOS results. It’s the difference between hope and certainty. And that’s worth every penny.
Bryson Carroll
November 29, 2025 AT 14:48Linda Rosie
December 1, 2025 AT 12:55Stability testing ensures patient safety. Regulatory compliance is non-negotiable.
Ross Ruprecht
December 3, 2025 AT 03:51So… how many of these chambers are just sitting there collecting dust? I bet half the labs are running 20% capacity. Why not just share them? Like, Uber for stability testing? Someone’s gotta make this less stupid.