When your prescription switches from the brand-name drug you’ve been taking to a generic version, you might feel uneasy. What if it doesn’t work the same? What if something changes inside the pill? You’re not alone. But here’s the truth: if it’s an authorized generic, you’re getting the exact same medication-down to the last ingredient-just without the brand name on the bottle.
What Exactly Is an Authorized Generic?
An authorized generic isn’t just another generic. It’s the exact same drug made by the original brand company, sold under a different label. Think of it like a car made in the same factory by the same workers, but with the logo removed. The active ingredients, the fillers, the coating, the shape, the size-all of it matches the brand-name version. The only difference? The packaging says nothing about the brand. And the price? Usually 20% to 40% lower. The FDA requires these to be listed in its Orange Book, and they’re tracked quarterly. In fact, about 20-25% of brand-name drugs with generic competition have an authorized generic version. And when they launch, prices drop faster and deeper than with traditional generics alone. Studies show retail prices fall by 4-8%, and wholesale prices drop 7-14% when an authorized generic enters the market.Why Switch? The Real Cost Difference
Let’s say you’re paying £80 a month for your brand-name blood pressure pill. Your insurance plan doesn’t cover it fully, so you’re paying out of pocket. Then, the authorized generic hits the shelves. Suddenly, your copay drops to £45. Or sometimes, it’s even lower-£30. That’s £600 saved a year. For someone on multiple medications, that adds up fast. Medicare Part D data from 2022 shows that when an authorized generic is available, 80-90% of prescriptions are automatically switched at the pharmacy. That’s not because pharmacists are pushing it-it’s because the insurance plan requires it. And the reason? Cost savings. The system is designed to steer you toward the most affordable option that’s just as effective. But here’s the catch: not all generics are authorized generics. A regular generic is made by a different company, even if it’s FDA-approved. It has the same active ingredient, but the inactive ones-like dyes or binders-can vary. That’s usually fine. But for some people with sensitivities, even small changes in fillers can cause issues. With an authorized generic, that risk disappears.How to Tell If It’s an Authorized Generic
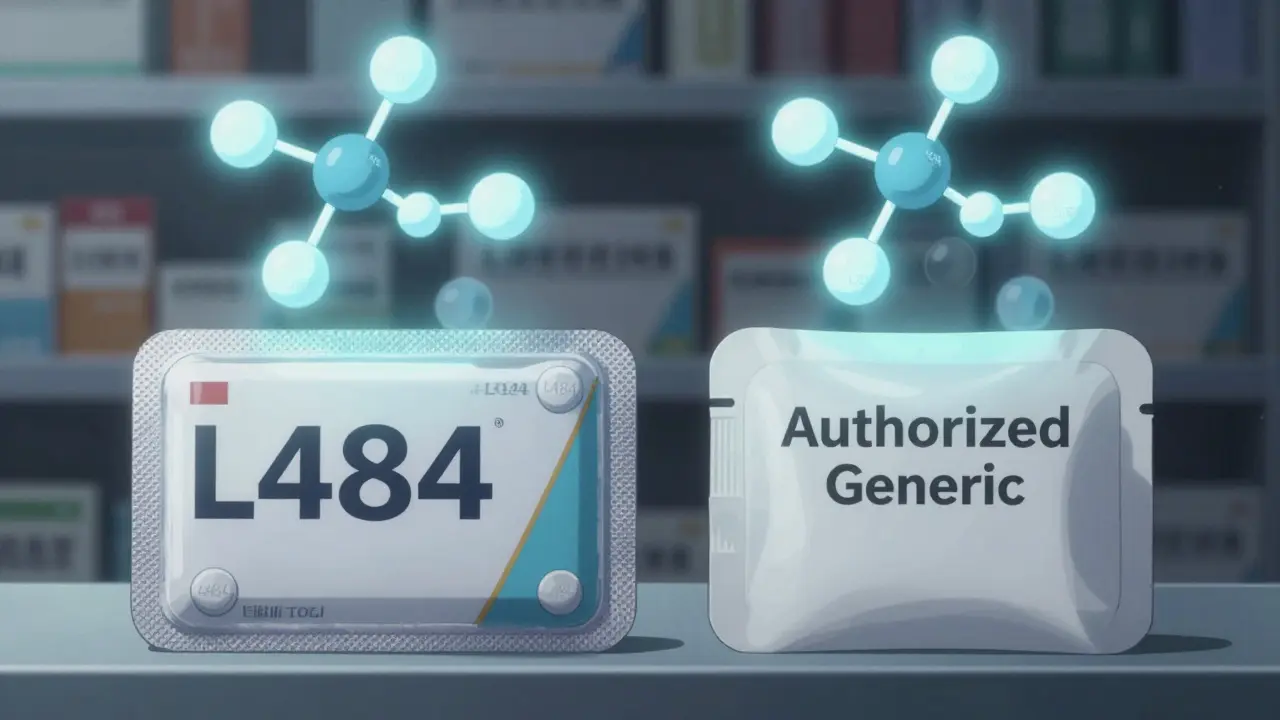
You can’t tell just by looking at the pill. The color or shape might be different, even if it’s the same drug. That’s because the brand company sometimes uses a different capsule shell or coating for the generic version. But that doesn’t mean it’s less effective. The only way to be sure is to check the FDA’s quarterly list of authorized generics. Go to the FDA’s website, search the Orange Book, and look up your drug. If you see a listing that says “Authorized Generic” under the brand name, you’ve found it. Your pharmacist can also confirm this-just ask: “Is this the authorized generic version?” Some pharmacies now flag authorized generics in their systems. If you use a large chain like Boots or Superdrug, the pharmacist might already know. But don’t assume. Always double-check.
What to Expect When You Switch
Most people notice zero difference. That’s because there isn’t one. The drug works the same. Your body processes it the same. Your blood levels stay stable. A 2018 study published in Health Affairs found no difference in hospitalization rates or medication discontinuation between patients who switched to authorized generics versus those who stayed on brand. But some patients do report confusion. If your pill used to be a blue oval and now it’s a white capsule, you might panic. That’s normal. But it’s not a sign something’s wrong. It’s just packaging. Pharmacists say about 65% of patient questions about generics come down to appearance. The fix? Simple: ask for the pill’s imprint code. Every pill has a unique marking-like “L484” or “20M.” Look it up on a pill identifier tool. If the code matches the brand, you’re good. If you’re worried about side effects, give it a week. Your body doesn’t need time to adjust to a new active ingredient-it’s the same. But if you’re switching from a brand you’ve taken for years, your brain might think something’s off. That’s psychological, not medical.Insurance and Pharmacy Hurdles
Your insurance plan will likely force the switch automatically. That’s good for your wallet. But if you’re on a specialty medication-like for multiple sclerosis, rheumatoid arthritis, or certain cancers-the process might need extra steps. Some plans require prior authorization even for authorized generics. That means your doctor has to call in a form saying the switch is safe. If you get a rejection notice, don’t panic. Call your pharmacy. They can help you appeal. Often, all they need is a note from your doctor saying, “No medical reason to avoid substitution.” Also, make sure your pharmacy updates your records. If you’re on multiple meds, especially with complex regimens, having the right version logged matters. Ask the pharmacist to note in your file: “Switched to authorized generic of [brand name].” That way, if you see a different provider, they won’t accidentally prescribe the brand again.When Not to Switch
There are rare cases where switching-even to an authorized generic-might not be ideal. If you’ve had a documented allergic reaction to a specific inactive ingredient in the past, check the label. Authorized generics use the same inactive ingredients as the brand, so that’s safe. But if you’ve had a reaction to a traditional generic, and you’re being switched to an authorized one, you’re likely fine. Still, if you’ve ever had a severe reaction, talk to your doctor first. Also, if you’re on a medication with a very narrow therapeutic window-like warfarin, lithium, or certain seizure drugs-your doctor might prefer you stay on the same version for consistency. Even with authorized generics, some providers feel more comfortable keeping patients on the exact same formulation. That’s not because the generic is unsafe-it’s just extra caution.
What to Do Next
If you’re considering switching, here’s your simple action plan:- Check your current prescription. Is it brand-name? If yes, ask your pharmacist: “Is there an authorized generic available?”
- Go to the FDA’s website and search the Orange Book. Look up your drug by name. See if “Authorized Generic” is listed.
- Ask your doctor: “Is it safe to switch to the authorized generic?” Most will say yes.
- When you get your refill, check the pill imprint and compare it to the brand version online. If it matches, you’re good.
- Keep your pharmacy updated. Let them know if you notice any changes in how you feel-even if you think it’s nothing.
Final Thought: It’s the Same Drug, Just Cheaper
You don’t have to choose between cost and quality. With an authorized generic, you get both. The FDA, the FTC, and years of real-world data confirm it: these drugs are identical. The only difference is the price tag. Millions of people in the UK and the US switch to authorized generics every year without issue. The fear comes from misunderstanding, not science. If your doctor says it’s safe-and they almost always will-then trust the process. Your wallet will thank you. And your body? It won’t even notice the change.Are authorized generics as safe as brand-name drugs?
Yes. Authorized generics are made by the same manufacturer, in the same facility, using the exact same formula as the brand-name drug. They are not just similar-they are identical. The FDA requires them to meet the same strict quality standards. There is no difference in safety, effectiveness, or how your body processes the medication.
Why does my authorized generic pill look different from the brand?
Even though the active ingredients are identical, the brand company may use a different capsule shell, color, or coating for the authorized generic version. These are inactive ingredients-fillers or dyes-that don’t affect how the drug works. The pill’s imprint code (the letters or numbers stamped on it) will match the brand version. You can verify this using the FDA’s pill identifier tool.
Can I ask my pharmacist to give me the brand instead of the authorized generic?
Yes, you can request it. But your insurance plan may not cover it, or you may have to pay the full brand price out of pocket. Most plans automatically substitute authorized generics because they’re cheaper and equally effective. If you insist on the brand, you’ll likely pay significantly more-sometimes double or triple the cost.
Do authorized generics work as quickly as brand-name drugs?
Yes. Because they are made with the same ingredients and manufacturing process, they dissolve and absorb into your bloodstream at the exact same rate. There is no delay or difference in how fast they start working. Studies tracking blood levels of patients switching to authorized generics show no change in absorption time or peak concentration.
Is there a list I can check to see which drugs have authorized generics?
Yes. The FDA maintains a publicly accessible, quarterly updated list of all authorized generics in the Orange Book. You can search by drug name on the FDA’s website. Many pharmacy systems now integrate this list, so your pharmacist may already know if an authorized generic is available when filling your prescription.
Why do some doctors hesitate to switch patients to authorized generics?
Most doctors fully support the switch. But a small number may have outdated concerns, especially if they’re used to prescribing brand-name drugs for decades. Some worry about patient anxiety or rare cases where inactive ingredients might affect someone with allergies. But since authorized generics use the exact same inactive ingredients as the brand, these concerns are usually unfounded. If your doctor is hesitant, ask them to check the FDA’s data-they’ll often change their mind.
Winni Victor
December 24, 2025 AT 20:54Okay but what if the authorized generic tastes like burnt plastic and gives me nightmares? I swear my last one made my left toe tingle for three days. Not placebo-my cat noticed. Also, why does the bottle say 'Made in China' now? That’s not the same factory. They swapped out the workers too. I’m not taking it. I’d rather pay double and sleep at night.
Linda B.
December 25, 2025 AT 18:21The FDA is a puppet of Big Pharma and the DEA. Authorized generics? Please. They’re testing subliminal messaging in pill coatings. The imprint code? It’s a tracking chip. You think they’d let you save money unless they were harvesting your biometrics. I’ve seen the documents. They’re not identical. They’re engineered. The ‘same formula’ is a lie wrapped in a regulatory bow.
Oluwatosin Ayodele
December 26, 2025 AT 09:05Y’all are overcomplicating this. In Nigeria, we take whatever generic works because we can’t afford brand names. If it’s FDA-approved and the imprint matches, it’s fine. Your body doesn’t care about branding. It cares about the molecule. If you’re having side effects, it’s not the generic-it’s your anxiety. Stop watching YouTube videos and check the actual FDA database. Simple.
Jason Jasper
December 26, 2025 AT 14:27I switched to an authorized generic for my thyroid med last year. I was nervous too-had been on the brand for 8 years. Took a week to adjust mentally. Physically? Nothing. Pill looked different, sure. But the imprint was identical. My TSH levels stayed perfect. I saved $500 a year. No drama. Just science.
Mussin Machhour
December 26, 2025 AT 16:36Yo if you’re still paying full price for brand-name pills in 2025 you’re leaving money on the table. Authorized generics are the ultimate flex-same drug, same results, same doctor approval, and you’re out $600 a year. That’s a weekend trip. A new pair of shoes. A month of coffee. Stop letting marketing fool you. Your body doesn’t care if it says ‘Pfizer’ on the bottle. It just wants the medicine to work.
Carlos Narvaez
December 28, 2025 AT 13:05Authorized generics are not ‘identical.’ They are bioequivalent. There is a difference. The FDA’s standards are minimal. You’re not getting the same manufacturing rigor. The brand invests in consistency. Generics? They optimize for cost. Don’t mistake ‘legal’ for ‘superior.’
Harbans Singh
December 29, 2025 AT 17:39Hey everyone, I’ve been a pharmacist for 15 years and I’ve seen this play out a thousand times. People freak out because the pill changed color. But the imprint code? Always matches. I show patients the FDA’s Orange Book on my tablet right there in the pharmacy. Half the time, they’re just scared of change. The other half? They’re relieved they can afford their meds. It’s not magic. It’s just smart. And yeah, I’ve had zero reports of adverse effects from authorized generics. Not one.
Justin James
December 31, 2025 AT 13:43Let me tell you what they don’t want you to know. The authorized generic is made in the same factory? Sure. But that factory is owned by the brand company, which also owns the patent, which means they control the supply chain, which means they can manipulate the fillers to make you dependent on the brand again later. They’re lowering the price now to get you hooked on the generic, then when the patent expires fully, they’ll raise it again. It’s a trap. I’ve seen the internal emails. They’re calling it ‘The Switch-and-Squeeze.’ And the FDA? They’re complicit. They’re funded by pharma. You think they’d expose this? No way. You’re being played. Save your money? No. Save your health. Demand the brand. Even if it costs more. Because they’re not giving you a deal-they’re setting you up.
Rick Kimberly
January 1, 2026 AT 03:40While the empirical evidence supporting bioequivalence is robust, one must acknowledge the psychological dimension of pharmaceutical adherence. The placebo effect, though non-physiological, exerts measurable influence on clinical outcomes. For patients with chronic conditions, the perceptual continuity of a familiar pill form may enhance compliance. Therefore, while the authorized generic is pharmacologically indistinguishable, its psychological discontinuity may, in select populations, constitute a clinically relevant variable. Prudent clinicians should consider patient preference as a component of therapeutic success.