When a product leaves a factory, it should work exactly as promised. No broken parts. No wrong labels. No hidden flaws. That’s not luck. It’s quality control testing - a structured, repeatable system built into every stage of manufacturing. Whether you’re making a smartphone, a car part, or a pill, skipping QC isn’t just risky - it’s expensive. A 2022 ASQ report found companies with solid QC systems cut scrap and rework costs by nearly a third. The difference between a product that sells and one that gets recalled often comes down to how well you test it - and when.
Define the Standards Before You Start
You can’t test for something if you don’t know what you’re looking for. The first step in any quality control process is defining clear, measurable standards. This isn’t vague stuff like “make it good.” It’s specific: “The outer casing must have a surface roughness (Ra) of 1.6 μm ± 0.3 μm.” Or “The active ingredient in each tablet must be within 95-105% of the labeled dose.” These standards come from product specifications, customer requirements, and industry regulations. In pharmaceuticals, that means FDA 21 CFR Part 211. In electronics, it’s IPC-A-610. In automotive, ISO/TS 16949. Every tolerance matters. A color shift measured at ΔE > 2.0 on the CIELAB scale might seem tiny to the naked eye, but in medical devices or consumer electronics, it’s a reject. Write these down. Make them visible on the shop floor. If your team can’t read and understand the standard in five seconds, it’s not good enough.Implement the Right Tools and Methods
Once you know what to check, you need the right tools to check it. This isn’t about buying the most expensive gear - it’s about matching the tool to the task. For dimensional checks, you might use digital calipers, CMM machines, or laser scanners. For electrical components, multimeters and automated test equipment (ATE) verify resistance, voltage, and continuity within ±10% tolerance. Chemical composition? Spectroscopy per ASTM E415. Surface finish? Gloss meters and profilometers. In-process quality control (IPQC) means checking at key points during production - not just at the end. A phone manufacturer might inspect solder joints after reflow, test battery voltage after assembly, and verify screen calibration before final packaging. Sampling isn’t random guessing. It follows AQL standards: MIL-STD-105E sets 0.65% defect tolerance for major issues in electronics. For critical safety parts - like airbag sensors or insulin pumps - you inspect 100%. No exceptions.Train Your People Like Experts
No machine works well without someone who understands it. Training is where most QC systems fail. A 2022 ASQ survey found 68% of manufacturing facilities struggled with inconsistent operator adherence. Why? Because operators weren’t trained properly - or they were trained once and never checked again. Effective training isn’t a one-hour PowerPoint. It’s hands-on. It’s shadowing. It’s passing a certification test. At NexPCB, operators go through 24-40 hours of role-specific training before touching equipment. They learn how to use calipers, how to interpret X-bar charts, how to document deviations. Certification rates target 95% or higher. And it’s not a one-time thing. Quarterly refreshers, audit feedback, and real-time coaching keep skills sharp. When an operator sees a defect, they should know whether it’s a one-off or a sign of a deeper problem. That’s expertise - not just following a checklist.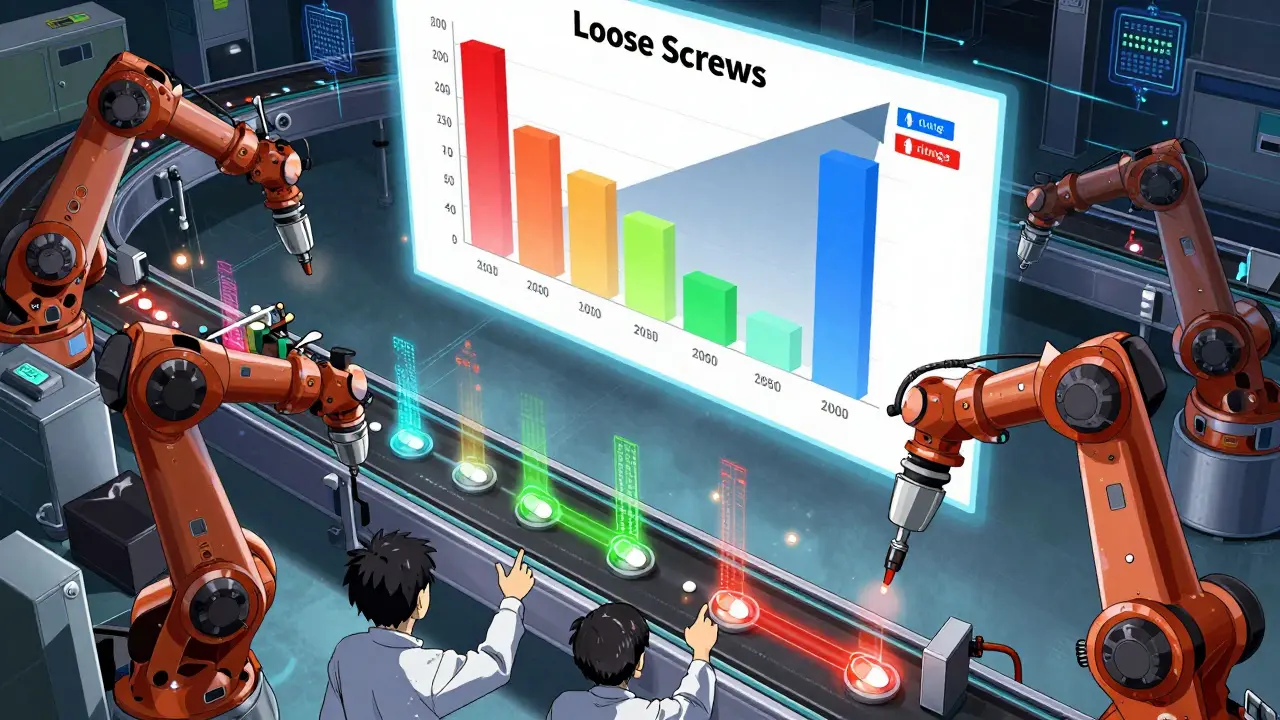
Monitor Everything in Real Time
Waiting until the end of the line to find a defect is like checking your car’s brakes after the crash. Modern QC uses real-time data collection. Sensors on machines track temperature, pressure, vibration. Vision systems scan for missing components or misaligned parts. IoT devices send alerts the moment a dimension drifts outside tolerance. Statistical process control (SPC) turns data into action. X-bar and R charts show if your process is stable. If the average weight of 100 pills jumps from 250mg to 258mg over an hour, the system flags it. Cpk values above 1.33 mean your process is capable - it fits comfortably inside the tolerance band. Below that? You’re playing Russian roulette with quality. Companies like Siemens use this in their Amberg plant. Real-time sensor data cuts defect detection time by 27%. That’s not magic. That’s visibility. When you see the problem as it happens, you fix it before 100 more units go bad.Analyze the Data - Don’t Just Collect It
Collecting data is easy. Making sense of it? That’s the hard part. Many manufacturers dump numbers into spreadsheets and call it done. That’s not analysis. That’s storage. True analysis means asking: Why did this happen? Use software like Minitab or JMP to run capability studies, Pareto charts, and root cause analysis. If 70% of defects are the same type - say, a loose screw on circuit boards - you don’t just tighten them. You investigate the torque tool. Is it calibrated? Is the operator using the right setting? Was the screw batch defective? A 2023 study at a medical device maker found that 43% of FDA Form 483 observations were due to unvalidated test methods. That’s not a QC failure - it’s a data failure.
Act Fast, Fix Right
Finding a problem is only half the job. Fixing it properly is the other half. That’s where CAPA - Corrective and Preventive Action - comes in. A defect isn’t just fixed. It’s investigated. A root cause is found. A change is made. And it’s documented. In pharmaceuticals, every deviation must be investigated within 72 hours. The fix isn’t just “rework the batch.” It’s “the screwdriver torque setting was incorrectly programmed. Update the work instruction. Retrain all operators. Verify the fix with 50 new units.” This is where culture matters. If operators fear blame, they hide problems. If they’re rewarded for spotting issues early, they become your best QC asset. Dr. David Schwinn, an ASQ Fellow, says the best systems combine human observation with machine data. A machine says “out of spec.” A person says, “I saw the material look dull before the machine flagged it.” That insight saves millions.Why This Matters Beyond Compliance
Quality control isn’t just about avoiding fines from regulators. It’s about trust. When customers buy your product, they’re betting you care about the details. A single defective batch can destroy a brand - especially in pharma or medical devices. The EU’s MDR 2017/745 and FDA’s new Quality Management Maturity initiative aren’t just checking boxes. They’re asking: Do you have a quality culture? The cost of poor quality is real. Automotive manufacturers spend 5.8% of revenue on QC. Consumer goods spend 3.2%. But the cost of a recall? That’s 10x that - and it’s not just money. It’s reputation. It’s lost customers. It’s legal battles. Meanwhile, companies using AI-powered visual inspection are seeing defect escape rates drop by 63%. Digital twins predict assembly flaws before they happen. Blockchain keeps quality records tamper-proof. These aren’t futuristic ideas - they’re being used now. But the foundation? Still the same: define, measure, analyze, act. That’s the Deming legacy. And it’s not going away.What’s Next for Quality Control
The future of QC isn’t replacing people with robots. It’s giving people better tools. By 2026, 65% of manufacturers will use real-time IoT data for quality decisions - up from 28% in 2022. AI will handle pattern recognition. Humans will handle context. Augmented reality glasses will guide inspectors through complex checks. Digital twins will simulate production runs before a single part is made. But here’s the catch: none of it works without solid basics. A fancy camera won’t help if your operators don’t know what a bad solder joint looks like. A blockchain ledger won’t fix a poorly calibrated torque tool. The most resilient systems blend cutting-edge tech with the timeless principles of clear standards, trained people, and fast, honest action.What’s the difference between quality control and quality assurance?
Quality assurance (QA) is about building quality into the process - planning, training, documentation, and system design. Quality control (QC) is about checking the output - inspecting, testing, and measuring finished or in-process products. QA asks, “Are we doing the right things?” QC asks, “Are we doing them right?” Both are needed. You can’t QC your way to quality if the system is broken from the start.
How often should QC equipment be calibrated?
Calibration frequency depends on the tool, usage, and industry. Digital calipers in a high-volume electronics line might need monthly checks. A CMM machine in a medical device plant could require quarterly calibration. Always follow the manufacturer’s guidelines and internal procedures. The FDA cited inadequate calibration in 41% of its 2021 warning letters. If you can’t prove your tools are accurate, your test results are meaningless.
Can small manufacturers afford proper QC testing?
Yes - but you start smart. You don’t need a $500,000 CMM machine on day one. Begin with basic tools: digital calipers, go/no-go gauges, and a simple checklist. Focus on critical points - the ones that cause returns or safety issues. A small manufacturer under 50 employees can set up a functional QC system in 4-8 weeks. The key is consistency, not complexity. A well-trained operator with a $200 tool is more valuable than a $50,000 machine gathering dust.
What’s the biggest mistake companies make in QC?
Over-relying on sampling without understanding the process. A 2023 internal analysis at NexPCB showed that 22% more defects slipped through when teams trusted statistical sampling over real-time observation. Sampling tells you what’s wrong - it doesn’t tell you why. If your process is drifting, you need to see it live. Combine AQL sampling with operator feedback and real-time data. Don’t just count defects. Understand them.
Is ISO 9001:2015 enough for quality control?
ISO 9001:2015 sets the baseline for a quality management system - it’s necessary, but not sufficient. It tells you to have a system. It doesn’t tell you how to test a pill’s potency or calibrate a torque screwdriver. You still need industry-specific standards: FDA 21 CFR Part 211 for pharma, IPC-A-610 for electronics, IATF 16949 for automotive. ISO 9001 is the framework. The rest is the content. Use it as a starting point, not the finish line.
James Dwyer
January 29, 2026 AT 05:19Finally, someone laid out QC like it actually works in the real world - not some corporate buzzword bingo. This isn’t about audits. It’s about not shipping broken shit to people who trusted you.
Every line here? Valid. Every metric? Necessary. No fluff.
Irebami Soyinka
January 29, 2026 AT 09:44Y’all in the West act like QC is some magical Western invention. We’ve been doing this in Nigeria for decades - with half the tools and ten times the grit. Your CMM machines? Cute. Our operators? They can eyeball a solder joint and tell you if it’s gonna fail in 3 months. No sensors needed.
Stop acting like quality is something you buy. It’s something you build - even with a hammer and a prayer.
Katie Mccreary
January 30, 2026 AT 16:02So let me get this straight - you’re saying if your operator can’t read a caliper, you’re doomed?
Wow. Groundbreaking.
matthew martin
January 31, 2026 AT 21:04This is the kind of post that makes you wanna go back to your job and high-five the guy who actually checks the torque on the damn screws.
I’ve seen factories where QC is a checklist someone fills out while scrolling TikTok. This? This is the antidote. Real people. Real tools. Real consequences.
And yeah - that bit about the dull-looking material before the machine flagged it? That’s the magic. Machines see numbers. Humans see stories. The best QC systems let both talk.
Also, I now want a pair of AR glasses that tell me if my coffee cup is properly glazed. Just saying.
Kathy Scaman
February 1, 2026 AT 01:41I work in a 20-person shop and we use go/no-go gauges and a whiteboard. We don’t have IoT. We don’t have digital twins. But we have one rule: if it doesn’t look right, it’s not going out.
Turns out, that’s 90% of QC.
jonathan soba
February 2, 2026 AT 06:22Let’s be honest - 80% of this is just rebranding common sense as ‘industry best practices.’
‘Define standards’? Shocking. ‘Train your people’? Revolutionary. ‘Don’t rely only on sampling’? Groundbreaking insight from 1998.
And yet, here we are, treating this like a TED Talk on quantum physics. The truth? Most companies don’t fail because they lack tools. They fail because they lack accountability.
And no, putting up a poster with ‘Cpk > 1.33’ doesn’t fix a culture of indifference.
Rose Palmer
February 4, 2026 AT 02:08While the foundational principles outlined are indeed sound and align with ISO 9001:2015 Clause 8.5.1, it is imperative to recognize that the implementation of Statistical Process Control (SPC) must be validated through documented procedures in accordance with IATF 16949:2016 Section 8.5.1.2. Failure to maintain calibration records traceable to NIST standards, as referenced in ISO 10012, constitutes a nonconformity that may trigger regulatory action under FDA 21 CFR Part 820.30.
Furthermore, the integration of AI-driven visual inspection systems must be accompanied by a risk-based validation protocol per ISO 13485:2016. Without this, the claim of a 63% reduction in defect escape rates lacks empirical substantiation and may be considered misleading.
Kevin Kennett
February 5, 2026 AT 01:34Man, I’ve been in shops where the QC guy was the only one who showed up on time. The rest? They’d rather nap than check a torque setting.
This post? It’s the wake-up call the whole industry needs. Training isn’t a box to tick. It’s the difference between a product that lasts and one that gets returned with a five-star review that says ‘broke after 2 days.’
And yeah - the guy who sees the dull material before the machine? That’s your MVP. Pay him more. Listen to him. He’s not just fixing defects - he’s saving your brand.
Sue Latham
February 5, 2026 AT 22:28Oh, sweet summer child. You think defining Ra 1.6 μm ± 0.3 μm is ‘clear’? That’s basic. Real QC professionals are measuring surface energy via contact angle goniometry and correlating it to adhesion failure in accelerated aging tests. You’re talking about kindergarten-level tolerances.
And don’t get me started on ‘AQL sampling.’ If you’re not doing 100% inspection on Class III medical devices, you’re not just negligent - you’re criminally irresponsible. Did you even read the MDR? Or are you just quoting ASQ like it’s scripture?
Also, blockchain? Cute. Have you tried auditing a decentralized ledger with a 20-year-old ERP system? Good luck with that.
Howard Esakov
February 7, 2026 AT 04:16AI visual inspection? 🤖✨
Oh wow. You mean… machines can now see what humans can’t? Like… a missing screw? Or a misaligned label?
Next you’ll tell me water is wet and gravity exists. I’m so proud of us.
Also, I’m 37 and still don’t know what Cpk means. But I like the graph. 📈
Bryan Fracchia
February 8, 2026 AT 11:30There’s something beautiful about this - how the same principles that kept ancient Chinese porcelain flawless are still what keeps your phone from exploding.
It’s not about tech. It’s about care. The guy who checks the torque because he knows someone’s kid might be using that car seat. The woman who notices the color shift because she remembers her sister’s allergic reaction to a bad batch of pills.
That’s not QC. That’s humanity.
And no algorithm will ever replace that.
Jess Bevis
February 10, 2026 AT 08:30My cousin runs a small PCB shop in Detroit. They use a $150 digital caliper and a whiteboard. No software. No sensors. But they have a rule: if you’re not proud of it, don’t ship it.
They’ve had zero recalls in 12 years.
Quality isn’t expensive. Indifference is.
Chris Urdilas
February 11, 2026 AT 11:51So let me get this straight - you’re telling me if you don’t have a chart with a line going up and down, you’re basically playing Russian roulette with a baby’s life?
Wow. That’s… oddly specific.
Also, I now have a new tattoo idea: ‘Cpk > 1.33’ on my bicep. For… science.
Phil Davis
February 11, 2026 AT 19:27Everyone’s talking about AI and digital twins like they’re magic.
Meanwhile, in the real world, someone’s still using a flashlight and a magnifying glass to check solder joints because the camera system keeps misreading the glare.
Tech doesn’t fix culture. People do.
And if your culture says ‘get it done,’ no algorithm will save you.
Timothy Davis
February 12, 2026 AT 19:14Let’s be real - you’re all just parroting ASQ and ISO without understanding the actual science behind the standards. You mention ‘Cpk > 1.33’ like it’s a gospel, but do you even know how to calculate it correctly when your data is non-normal? Did you test for autocorrelation in your SPC charts? Or are you just using Minitab’s default settings like a toddler with a calculator?
And ‘100% inspection for airbag sensors’? That’s not even the right approach. You should be using reliability-based sampling with Weibull analysis, not blind faith. You’re all just doing QC theater.