Long COVID Treatment Risk Calculator
Recommended Treatment Options
Detailed Comparison
By December 2025, over 200 million people worldwide are living with symptoms that won’t go away after a COVID-19 infection. Fatigue so deep it feels like your bones are made of lead. Brain fog that makes simple conversations feel like solving a puzzle. Heart palpitations after walking up a flight of stairs. These aren’t rare side effects-they’re the new normal for millions. And yet, there’s still no FDA-approved drug to treat Long COVID. That’s not just frustrating. It’s dangerous.
People are desperate. They’re trying anything: off-label prescriptions, supplements, experimental trials. But without clear guidelines, they’re navigating a minefield. Some meds help. Others make things worse. And we’re still guessing at what’s safe, what works, and why.
Baricitinib: A Promising Drug With Serious Risks
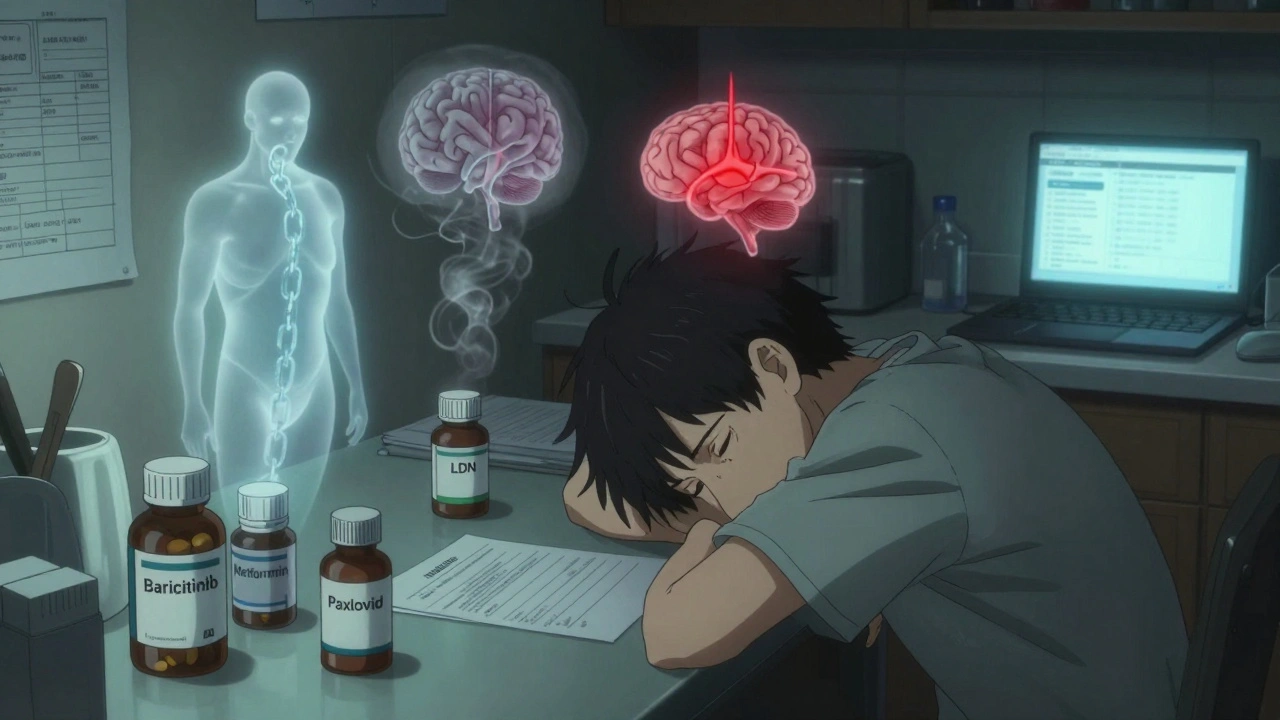
Baricitinib is one of the most studied drugs for Long COVID right now. It’s a JAK inhibitor, originally approved for rheumatoid arthritis and alopecia. It worked in hospitalized COVID patients by calming an overactive immune response. That’s why researchers are now testing it for Long COVID-where immune dysfunction is suspected.
The REVERSE-LC trial, led by Vanderbilt and funded by the NIH, is enrolling over 1,000 patients. Results are due in late 2026. Early signs are encouraging: patients report less fatigue and brain fog. But the risks? They’re real. In rheumatoid arthritis trials, 10-20% of patients got serious infections. Some developed lymphoma. Others had heart attacks or blood clots. That’s not a small trade-off. Long COVID patients are often younger and healthier than rheumatoid arthritis patients. Giving them a drug that suppresses immunity could backfire badly.
Doctors are split. Some say the benefits outweigh the risks. Others warn that Long COVID patients may already have immune imbalances-adding an immunosuppressant could trigger something worse. No one knows yet.
Metformin: The Unexpected Hero
Here’s one you might not expect: metformin. The cheap, old diabetes drug is now showing real promise for preventing Long COVID. A 2023 University of Minnesota trial published in Nature Medicine found that people who took metformin within five days of a positive test had a 41% lower chance of developing Long COVID.
That’s huge. And it’s not just theoretical. Real people in the trial saw fewer symptoms months later. But here’s the catch: 36% of those taking metformin had serious stomach issues-nausea, vomiting, diarrhea. For someone already battling fatigue and brain fog, that’s brutal. Many dropped out. Still, it’s the strongest evidence we have so far for a treatment that might actually stop Long COVID before it starts.
Doctors are now prescribing it off-label for early COVID patients. But there’s no official guidance. No dosing standard. No way to know who will benefit-and who will suffer.
Low-Dose Naltrexone: Hope With Side Effects
Low-dose naltrexone (LDN) is another off-label favorite. Typically used for opioid addiction at 50 mg, LDN uses 1-5 mg daily. It’s thought to calm inflammation and reset the nervous system.
A 2024 study from Nova Southeastern University followed 120 Long COVID patients. Sixty-two percent reported less fatigue. Forty percent said their brain fog improved. But 28% had trouble sleeping. 19% got headaches. A few reported mood swings. These aren’t rare. They’re common. And they’re not always mild.
LDN isn’t approved for Long COVID. No one knows the right dose. No one knows how long to take it. But because it’s cheap and available, people are using it anyway. And many are reporting it’s the only thing that helps.
Paxlovid: Mixed Results, Bitter Taste, and Hidden Risks
Paxlovid, the antiviral combo of nirmatrelvir and ritonavir, was a game-changer for acute COVID. But for Long COVID? The data is messy.
A small UCSF study found 38% of patients improved after a 15-day course. But a much larger NIH trial found no difference between Paxlovid and placebo. That’s confusing. Why the contradiction? Maybe timing. Maybe dose. Maybe who you are.
Then there are the side effects. Nearly 80% of users reported a terrible, metallic taste-so bad some stopped taking it. Ritonavir also interferes with dozens of other medications. If you’re on blood thinners, statins, or even some antidepressants, Paxlovid could be dangerous. For someone with Long COVID who’s already juggling multiple symptoms and meds, that’s a major red flag.
Drugs That Failed: The Dark Side of Research
Not every trial gives hope. Some give warnings.
BC007, a drug designed to neutralize harmful autoantibodies, was halted in March 2025. In a phase II trial, 24.7% of patients had side effects-slightly more than the placebo group. Three serious infusion reactions happened in the treatment group. One was life-threatening. The trial was stopped.
Other drugs like AER002 and polymerized collagen are still being tested. Early safety looks okay. But they’re in small trials. We don’t know if they’ll work-or if they’ll cause harm later.

The Big Unknowns
Here’s what keeps researchers up at night:
- There’s no blood test for Long COVID. No biomarker. No way to know if you have it-or if a treatment is working.
- Long COVID isn’t one disease. It’s at least four different types: some driven by lingering virus, others by autoimmunity, some by nerve damage, others by metabolic chaos. One drug won’t fix them all.
- Safety data comes from people with different illnesses. Baricitinib’s risks were studied in older, immunocompromised patients. LDN’s effects were seen in chronic pain patients. But Long COVID patients are often young, previously healthy. We’re guessing how they’ll react.
- We don’t know how long to take these drugs. Weeks? Months? Years? What happens if you stop? What if you keep going?
Even the NIH admits it. Their own 2025 update said: "We have not yet identified reliable biomarkers to guide treatment." That’s not a delay. That’s a fundamental gap.
What Patients Are Actually Doing
A survey of 15,000+ Long COVID patients by the Body Politic Support Group found:
- 68% have tried at least one medication off-label
- 32% tried metformin
- 29% tried LDN
- 24% tried Paxlovid
But here’s the kicker: 57% said the meds didn’t help enough. 41% said the side effects were worse than their symptoms.
People aren’t waiting for approval. They’re trying things. And they’re getting hurt.
What’s Next?
The NIH’s RECOVER initiative is launching more trials in late 2025. New candidates include tirzepatide (Mounjaro), a diabetes and weight-loss drug that may help with brain fog and fatigue. Stellate ganglion blocks-once used for chronic pain-are being tested for anxiety and heart rate issues in Long COVID.
But none of these are guaranteed. And none are safe until proven so.
The first FDA-approved Long COVID treatment might come by 2027 or 2028. But until then, patients are on their own. Doctors are guessing. Researchers are racing.
And the biggest question remains: Who gets to decide what’s safe when no one knows what’s working?
Roger Leiton
December 3, 2025 AT 11:02Been on LDN for 6 months now. My brain fog lifted like a foggy windshield after a wiper pass 🤯 Not magic, but it’s the first thing that didn’t make me feel worse. Still get sleepy sometimes, but I’ll take it over collapsing after showering. 🙏
Laura Baur
December 3, 2025 AT 16:52It’s not merely frustrating-it’s a systemic failure of medical epistemology. We have no biomarkers, yet we prescribe immunosuppressants derived from arthritis protocols to a population that, by and large, was previously healthy and young. This is not medicine. It is pharmacological roulette with a loaded chamber. The FDA’s inertia is not bureaucratic delay-it is moral negligence masked as caution.
Metformin’s 41% reduction? That’s not a ‘hint.’ It’s a revelation. Yet we treat it like a folk remedy because it’s cheap and old. Meanwhile, baricitinib trials get NIH funding while a $0.10-per-dose drug sits on pharmacy shelves with no prescribing guidelines. Capitalism doesn’t care if you’re dying slowly-it cares if you can be monetized.
And don’t get me started on Paxlovid’s metallic taste. That’s not a side effect. That’s a psychological weapon. Imagine being told to swallow poison that makes your tongue feel like licking a battery, and then being told you’re ‘non-compliant’ because you quit.
We are not patients. We are test subjects in a clinical trial of societal indifference.
Arun kumar
December 4, 2025 AT 03:37bro i tried metformin for 2 weeks… my stomach was like a washing machine on spin cycle 😭 but i swear my energy was better after 10 days. i quit cause i couldn’t eat anything without feeling like i’d explode. still think it’s worth it if u can handle the guts.
Zed theMartian
December 5, 2025 AT 15:53Oh wow. So we’re just going to hand out JAK inhibitors like candy to people who didn’t even die from COVID? Brilliant. Next up: giving cancer chemo to people who had a cold. I’m sure the CDC will love this. 🙄
Meanwhile, the real cure? Rest. Sleep. Less stress. But that doesn’t sell drugs, does it? No. We need a $12,000-a-year miracle pill with a 20% chance of lymphoma. That’s the American dream.
Rebecca M.
December 5, 2025 AT 18:09Of course the only thing that helps is something no one can patent. 🙄
Metformin. LDN. Rest. Sleep. The things that cost nothing and require zero corporate sponsorship. Meanwhile, baricitinib gets a $2 billion trial because someone’s cousin works at Eli Lilly. 💅
And the taste of Paxlovid? That’s not a side effect. That’s God’s way of saying, ‘You’re not ready for this.’
Lynn Steiner
December 7, 2025 AT 10:00I just want to scream into the void. I’ve tried everything. Everything. And I’m tired. Not just ‘I stayed up too late’ tired. I’m ‘my bones are made of wet cement’ tired. And now I’m supposed to take more pills that might kill me? 😭
Who’s gonna fix this? When? I’m 32. I used to hike. Now I cry after brushing my teeth.
Alicia Marks
December 9, 2025 AT 00:49You’re not alone. Keep going. Small wins count. Even if it’s just getting out of bed. 💪
Paul Keller
December 10, 2025 AT 21:24While the pharmacological landscape remains fraught with uncertainty, it is imperative to recognize the emergent patterns in patient-reported outcomes. The efficacy of metformin, despite its gastrointestinal adverse effects, suggests a metabolic component to Long COVID pathophysiology that has been historically underexplored. Furthermore, the divergence in Paxlovid trial results may be attributable to heterogeneity in patient phenotypes-perhaps those with persistent viral reservoirs respond differently than those with autoimmune-driven symptoms. The absence of biomarkers does not negate biological reality; it merely indicates our diagnostic limitations. We must prioritize stratified trials and patient-centered outcome measures, rather than relying on population-level averages that obscure individual trajectories.
Steve Enck
December 12, 2025 AT 10:06The entire Long COVID discourse is a statistical mirage. You have a population of individuals with non-specific, subjective symptoms-fatigue, brain fog, palpitations-none of which are objectively quantifiable. You then administer drugs with known risks, observe subjective improvements in 30-60% of cases, and declare ‘promise.’ This is not science. This is placebo-driven confirmation bias amplified by social media anecdotes. The NIH is funding witchcraft dressed in lab coats.
Metformin? It’s a diabetes drug. LDN? A low-dose opioid antagonist. Paxlovid? An antiviral for acute infection. None were designed for this. You’re not treating a disease-you’re throwing spaghetti at the wall and calling it ‘research.’
And let’s not pretend patients aren’t desperate. Desperation does not equal evidence. It equals vulnerability. And the pharmaceutical industry smells blood.
Jay Everett
December 12, 2025 AT 11:45LDN changed my life. Like, seriously. I went from barely making it to the fridge to walking my dog around the block without needing a nap. 🌟
Yeah, I had weird dreams for the first week-like, ‘I was a sentient toaster arguing with a squirrel’ weird-but that faded. And the headaches? Took a couple days. But the brain fog? Gone. Not ‘better.’ Gone.
My doc didn’t know squat about it, so I had to Google it myself. Found a Long COVID Facebook group. Someone shared their dose. I started at 1.5mg. Took it at 9pm. Slept like a baby. Woke up feeling… human.
It’s not FDA-approved. But neither is hope. And right now? Hope comes in a little white pill.
PS: If you try it, go slow. And don’t drink alcohol. Trust me. 😅
मनोज कुमार
December 13, 2025 AT 07:05baricitinib risky metformin gut wreck ldn sleep issues paxlovid taste hell
no biomarkers no answers just guesswork
pharma will profit anyway
Joel Deang
December 14, 2025 AT 18:05so i tried metformin cause my friend said it worked… and yeah i got the stomach thing 😅 but also i started dreaming in color? like, vivid, weird, beautiful dreams. not sure if it’s the drug or just my brain finally relaxing. either way, i’ll take it. 🌈
also paxlovid tasted like licking a battery and crying. nope. not doing that again.
Jack Dao
December 16, 2025 AT 15:26Of course you’re all wasting your time. You think some random pill will fix a condition that’s likely rooted in spiritual imbalance or moral decay? The real issue isn’t medicine-it’s your lack of discipline. Get off the couch. Meditate. Eat clean. Stop complaining. You’re not sick. You’re lazy.
And don’t even get me started on LDN. That’s not science. That’s New Age nonsense dressed in pharmaceutical packaging.
Real people don’t need drugs. They need willpower.
Roger Leiton
December 18, 2025 AT 14:22@Jack Dao - I used to think that way too. Until I couldn’t walk to the mailbox without my heart pounding like a drum. I’m not lazy. I’m broken. And I’m trying everything to fix it. So if you’re gonna judge, at least come talk to me after you’ve lived it.
Also… I’ve been meditating for 2 years. I eat kale. I sleep 8 hours. And I still can’t get out of bed. So yeah. I’m taking the pill. And I’m not sorry.