When you pick up a prescription, you might see two options on the label: a brand name like Lipitor or a generic called atorvastatin. One costs hundreds of dollars. The other might be free with a coupon. But are they really the same? If you’re worried about your health, it’s natural to wonder: Is the cheaper version just as safe? Does it work just as well? The short answer is yes - for almost everyone, most of the time. But there are important details you need to know before making a switch.
What Makes a Drug "Generic"?
A generic drug isn’t a copycat. It’s the exact same medicine. The FDA requires that generic versions contain the same active ingredient, in the same strength, and delivered the same way - whether it’s a pill, injection, or cream - as the original brand-name drug. That means if your brand-name drug has 10 mg of atorvastatin, the generic has 10 mg of atorvastatin. No more, no less.
The big difference? The inactive ingredients. Things like fillers, dyes, or coatings that help the pill hold together or make it easier to swallow. These don’t affect how the medicine works, but they can cause rare reactions in people with allergies or sensitivities. That’s why some people notice a change in how a pill looks or tastes - but not how it affects their body.
Generics become available after the original patent expires. That’s why Lipitor, once sold for over $300 a month, now costs nothing in many cases. The same goes for Plavix (clopidogrel), Singulair (montelukast), and dozens of others. The system was designed by the Hatch-Waxman Act of 1984 to save money without sacrificing safety. And it works.
How Do We Know Generics Work the Same?
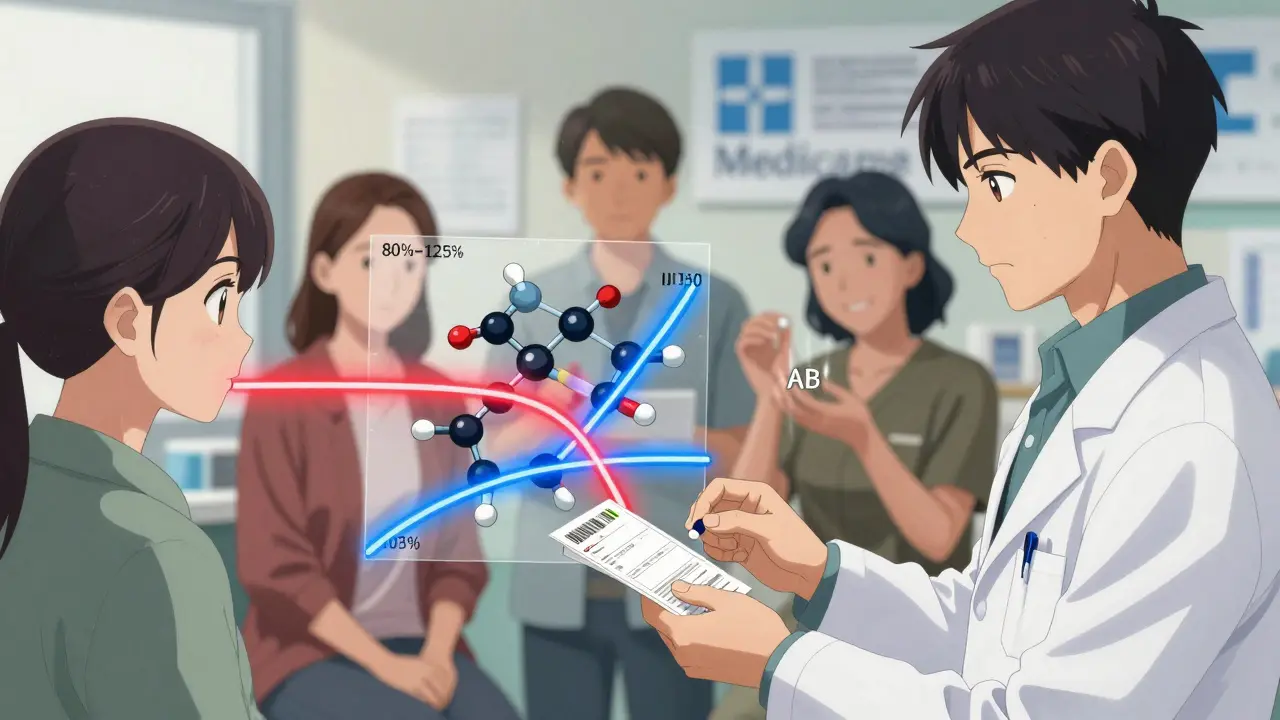
The FDA doesn’t just take a company’s word for it. To get approval, generic manufacturers must prove bioequivalence. That means they run clinical trials with 24 to 36 healthy volunteers, measuring how fast and how much of the drug enters the bloodstream.
The key numbers? The amount of drug absorbed over time (AUC) and the highest level reached (Cmax). The FDA says a generic is equivalent if these values fall within 80% to 125% of the brand-name drug. That’s not a 20% difference in active ingredient - it’s a 20% difference in how quickly the body absorbs it. Most studies show differences under 4%, and over 98% of generics show absorption differences under 10%.
That’s not just theory. A 2020 study in Scientific Reports tracked 1.2 million patients across 17 drug classes. The results? Generic versions were just as effective - and in 10 out of 17 cases, they led to fewer deaths. For heart disease drugs like statins and beta-blockers, the outcomes were identical.
Cost Difference? It’s Staggering
Let’s look at real numbers. In November 2023, a 30-day supply of brand-name Lipitor cost $335.37. The generic? $0.00 with a GoodRx coupon. Plavix? $450.22 versus $0.00. Even without coupons, generics typically cost 80% to 85% less.
In 2023, generics saved the U.S. healthcare system $373 billion. That’s not a guess. It’s from the Generic Pharmaceutical Association’s report. For patients, that means fewer skipped doses, fewer hospital visits, and less financial stress. In the UK, where prescriptions are often free or low-cost, the savings still matter - especially for long-term conditions like high blood pressure or diabetes.
Even with insurance, many plans push generics because they’re cheaper. Medicare Part D fills 92% of its prescriptions with generics. Commercial plans aren’t far behind at 87%.
When Should You Be Cautious?
Not all drugs are created equal - and not all substitutions are risk-free. Some medications have a narrow therapeutic index (NTI). That means even tiny changes in blood levels can cause serious problems - too little and the drug doesn’t work; too much and it becomes toxic.
These include:
- Warfarin (blood thinner)
- Levothyroxine (thyroid hormone)
- Phenytoin (anti-seizure)
- Lithium (mood stabilizer)
For these, the FDA recommends extra care. Blood tests within 7 to 14 days after switching can catch any shifts. The American Society of Health-System Pharmacists says this is standard practice. Yet, a 2023 survey found only 32% of doctors recognized levothyroxine as an NTI drug - even though it’s one of the most commonly switched medications.
That’s why many patients report issues after switching. On Reddit, 28% of users said they noticed changes - mostly with levothyroxine. Some felt more tired. Others had heart palpitations. These aren’t imaginary. Small variations in absorption can affect thyroid levels enough to cause symptoms. The fix? Talk to your doctor. Get a blood test. Don’t assume it’s "all in your head."
Why Do Some People Still Doubt Generics?
Despite the evidence, skepticism persists. A 2022 survey by Pharmacy Times found 43% of patients believed generics were less effective. One in four refused substitution when offered. Why?
- Marketing. Brand-name companies spend millions on ads that imply generics are inferior.
- Appearance. A different shape, color, or size feels "wrong," even if it’s identical inside.
- Personal experience. One bad switch can stick in your memory - even if 10 others went fine.
And yes, there are rare cases where a patient reacts to a new filler. That’s why pharmacists are trained to ask: "Have you had any issues with generics before?"
But here’s the truth: 89% of users in a GoodRx survey said they saw no difference in effectiveness. And for most drugs - antibiotics, blood pressure pills, antidepressants - the difference is statistically zero.

What Should You Do?
If you’re on a medication and your pharmacist switches you to a generic:
- Don’t panic. For 9 out of 10 drugs, it’s perfectly safe.
- Check the label. Is it an AB-rated generic? That’s the FDA’s highest bioequivalence rating.
- Watch for changes. If you feel different - more tired, dizzy, or anxious - note it. Don’t ignore it.
- Ask for a blood test. Especially if you’re on warfarin, levothyroxine, or similar drugs.
- Call your doctor. If you’re uncomfortable, ask if you can stay on the brand. But know: it’s usually not medically necessary.
And if you’re starting a new medication? Always ask: "Is there a generic?" Most of the time, the answer is yes - and it’s the better choice.
The Future: Better, Cheaper, Safer
The FDA approved 1,021 new generic drugs in 2023 - up 12% from the year before. Complex generics - like inhalers and topical creams - are finally catching up. A 2023 MIT study showed new testing methods could reduce absorption differences in warfarin generics to under 2%. That means even NTI drugs will become safer to switch.
Meanwhile, biosimilars - the next generation of generics for biologic drugs like Humira - are now approved in the U.S. and cutting costs by 15% to 30%. The pipeline is full. The science is solid. The savings are real.
What’s holding us back? Not science. Not safety. Just old habits and misinformation.
Are generic drugs as safe as brand-name drugs?
Yes, for the vast majority of medications. The FDA requires generics to contain the same active ingredient, strength, and delivery method as the brand-name version. They must also prove bioequivalence through strict testing. Studies involving millions of patients show no difference in safety or effectiveness for most drugs. The only exceptions are drugs with a narrow therapeutic index, where small changes in blood levels matter - like warfarin or levothyroxine. Even then, switching is safe with proper monitoring.
Why do generics cost so much less?
Brand-name drug companies spend years and billions developing a new medicine, including clinical trials and marketing. Once the patent expires, other manufacturers can make the same drug without repeating those costs. They don’t need to run new safety trials - just bioequivalence studies. That cuts production costs dramatically. Competition among multiple generic makers drives prices down further. That’s why a drug that cost $300 a month as a brand can drop to $0 with a coupon.
Can I switch from a brand-name drug to a generic safely?
For most drugs, yes - and it’s often recommended. Pharmacists are trained to substitute generics unless the prescription says "dispense as written." But for drugs with a narrow therapeutic index - like warfarin, lithium, or levothyroxine - your doctor may want to check your blood levels a week or two after switching. If you feel different after switching, don’t assume it’s psychological. Talk to your provider. A simple blood test can confirm whether the generic is working the same way.
Do generics have the same side effects as brand-name drugs?
The active ingredient causes the side effects - so yes, the same side effects should occur. But sometimes, the inactive ingredients (like dyes or fillers) in generics can cause rare reactions - like an upset stomach or rash - that didn’t happen with the brand. This isn’t about effectiveness. It’s about tolerance. If you notice a new reaction after switching, tell your doctor. It may just mean you need a different generic version.
How can I tell if my generic is FDA-approved?
Look for the "AB" rating on the prescription label or in the FDA’s Orange Book. AB-rated generics are considered fully interchangeable with the brand-name drug. If your pharmacy gives you a generic without an AB rating, ask why. Some older generics may have a "B" rating, meaning they’re approved but may not be interchangeable. Most generics today are AB-rated. If you’re unsure, ask your pharmacist to check the Orange Book - it’s publicly available and updated quarterly.
Final Thought
Choosing a generic isn’t about cutting corners. It’s about using science to save money - without sacrificing health. For the vast majority of people, the cheaper option works just as well. The data doesn’t lie. The savings are real. And the safety record? Stronger than most people think. If you’ve been hesitant, ask your doctor or pharmacist: "Is there a generic for this?" The answer might surprise you - and save you hundreds.
Jessica Klaar
February 8, 2026 AT 04:53I switched my levothyroxine to generic last year after my insurance dropped the brand. I was nervous as hell - I’d read all the Reddit threads about people feeling like zombies. But I followed the advice: got my TSH checked two weeks in. Numbers were perfect. I didn’t feel different. No fatigue, no heart palpitations. Honestly? I’m saving $400 a year and my thyroid is still chill. If you’re scared, just test it. No drama needed.
Also, side note: my pharmacist gave me a different pill shape. It looked like a alien artifact. But it worked. Don’t let the color freak you out.
PAUL MCQUEEN
February 8, 2026 AT 20:42Yeah, sure. FDA says they’re equivalent. But have you ever seen how they test this? 24 healthy volunteers? In a lab? That’s not real life. People with liver issues, gut problems, or who take five other meds? Nah. They don’t test those folks. And don’t get me started on how many generics come from India or China - no one’s auditing those factories. I’ll stick with my brand. My body’s not a guinea pig.
glenn mendoza
February 8, 2026 AT 22:26It is with profound gratitude for the rigorous scientific framework established by the Hatch-Waxman Act that I acknowledge the remarkable efficacy and safety profile of generic pharmaceuticals. The bioequivalence standards mandated by the Food and Drug Administration - specifically the 80% to 125% confidence interval for AUC and Cmax - represent not merely regulatory compliance, but a triumph of evidence-based medicine.
Moreover, the longitudinal data from peer-reviewed studies, including the 2020 Scientific Reports meta-analysis, affirm that generic substitution is not only statistically non-inferior, but in certain therapeutic classes, demonstrably superior in terms of patient adherence and mortality reduction. One must therefore conclude that resistance to generics is not grounded in pharmacology, but in psychological bias and misinformation propagated by corporate marketing.
For the conscientious patient, the path forward is clear: embrace the science, trust the data, and prioritize health over perception.
Kathryn Lenn
February 9, 2026 AT 10:08Let me guess - the FDA is just a front for Big Pharma’s secret plan to make you take pills made in a basement in Bangalore with chalk and hope.
Real talk? I switched to generic warfarin. Two weeks later, my INR spiked to 5.2. I almost bled out. They said it was "within tolerance." Tolerance? My blood was practically a waterfall. Meanwhile, my brand-name version? Stable for five years. Coincidence? Or is this just how they get you hooked on cheaper meds so they can sell you more expensive ER visits later?
And don’t even get me started on the dye in the pills. I think they’re adding fluoride. Or worse - microchips. I’ve seen the videos.
John Watts
February 9, 2026 AT 12:53Y’all are overthinking this. I’ve been on generic statins for 8 years. My cholesterol? Better than ever. My wallet? Thank you, Jesus. My doctor? High-fived me.
Generics aren’t "cheap versions." They’re the same damn medicine, just without the fancy logo and the CEO’s yacht fund. Stop treating your medication like it’s a luxury perfume.
And if you’re scared? Talk to your pharmacist. Ask them to check the AB rating. If it’s AB, you’re golden. If it’s not? Ask why. But don’t let fear cost you $300 a month for something that’s been proven to work just as well.
Health isn’t about brand names. It’s about consistency. And generics? They’re the quiet heroes of American healthcare.
Angie Datuin
February 10, 2026 AT 16:56I switched my antidepressant to generic last month. Felt kinda blah for a week. Then it was fine. Maybe it was the weather. Or my cat. Or I just needed time. Not a big deal. I didn’t panic. Just waited. It worked out.
Monica Warnick
February 11, 2026 AT 18:27I don’t trust generics. Not because I’m paranoid - because I’ve read the reports. The FDA doesn’t require full clinical trials. They just require "bioequivalence" - which means they don’t even have to test on people with actual diseases. Just healthy college kids. That’s not medicine. That’s a bet. And I’m not gambling with my life.
Plus, my generic levothyroxine has a weird smell. I swear it’s different. I’ve been on the same brand for 12 years. Why mess with perfection? Someone’s making money off this. And it ain’t me.
Ashlyn Ellison
February 12, 2026 AT 23:15My mom’s on warfarin. Switched to generic. Got her INR checked. Perfect. She’s been saving $200/month. She didn’t even notice the pill looked different. She’s 78. Doesn’t care about labels. Just wants to feel okay.
Meanwhile, I’m over here Googling "can generic lithium cause existential dread?"
Turns out, no. Just me. My brain’s the problem, not the pill.