Gene Therapy Drug Interaction Risk Checker
How this works
Gene therapy can alter your body's ability to process medications through immune reactions and enzyme changes. This tool estimates potential interaction risks based on the article's findings. Important: This is not a medical diagnosis and does not replace professional consultation.
Gene therapy isn’t just another treatment option-it’s a fundamental rewrite of how medicine works. Instead of managing symptoms with pills or injections, it aims to fix the root cause by delivering new genes into your cells. But this power comes with risks you won’t find in any drug label. When gene therapy meets regular medications, the consequences can be unpredictable, delayed, and sometimes deadly.
Why Gene Therapy Isn’t Like Other Drugs
Most drugs are temporary. You take a pill, it works for a few hours or days, then your body clears it out. Gene therapy is different. It’s designed to last. Some therapies insert new DNA into your cells permanently. Others use viruses that stick around for years, quietly producing therapeutic proteins. That permanence is the whole point-but it’s also the problem.When a viral vector delivers a gene to your liver, muscle, or neurons, it doesn’t just stop there. It can trigger immune reactions you didn’t sign up for. In 1999, 18-year-old Jesse Gelsinger died after a gene therapy trial using an adenovirus vector. His body overreacted to the virus, causing a cytokine storm that shut down his organs. The same vector had already killed two monkeys in preclinical tests. That tragedy changed everything. Today, regulators demand years of follow-up, not weeks.
How Viral Vectors Mess With Your Medications
The viruses used in gene therapy-like AAV, adenovirus, or lentivirus-are engineered to be harmless. But they’re still viruses. Your immune system recognizes them as invaders. That triggers inflammation, which doesn’t just affect your immune cells. It changes how your liver processes drugs.Your liver uses enzymes called cytochrome P450 to break down about 70% of all prescription medications. When a viral vector causes inflammation, those enzymes can speed up, slow down, or even shut down. Imagine taking your blood pressure pill as usual, but suddenly your body can’t metabolize it anymore. Your levels spike. You get dizzy. You might even have a stroke. Or worse-the enzymes go into overdrive, and the drug gets cleared too fast. Your treatment stops working.
This isn’t theoretical. In trials for AAV-based therapies targeting inherited blindness or muscle disorders, patients on common drugs like statins, antidepressants, or anticoagulants showed unexpected changes in drug levels. No one knew why. No standard test could predict it. Each patient’s immune response was different. So was their drug interaction risk.
Off-Target Effects: When Therapy Hits the Wrong Cells
Gene therapy isn’t precise enough yet. Vectors don’t always land where they’re supposed to. A therapy meant for the liver might also reach the spleen, kidneys, or even brain tissue. That’s bad enough. But if the therapy accidentally alters a gene in a cell that helps metabolize drugs-say, a liver cell that produces CYP3A4-now you’ve created a hidden variable in your drug response.Worse, some therapies involve removing cells from your body, editing them in the lab, and putting them back. These modified cells might behave differently once reinfused. They could migrate. They could multiply uncontrollably. In the early 2000s, five children treated for SCID-X1 with gamma-retroviral vectors developed leukemia. The therapy had inserted the new gene right next to a cancer-causing gene called LMO2. One child died. The vector didn’t just fix their immune system-it accidentally turned on a tumor switch.
Today, regulators require long-term monitoring for any therapy with integrating vectors. But even non-integrating therapies like AAV can cause problems years later. The gene keeps working. The immune system keeps reacting. And your body’s ability to handle other drugs? It’s still changing.
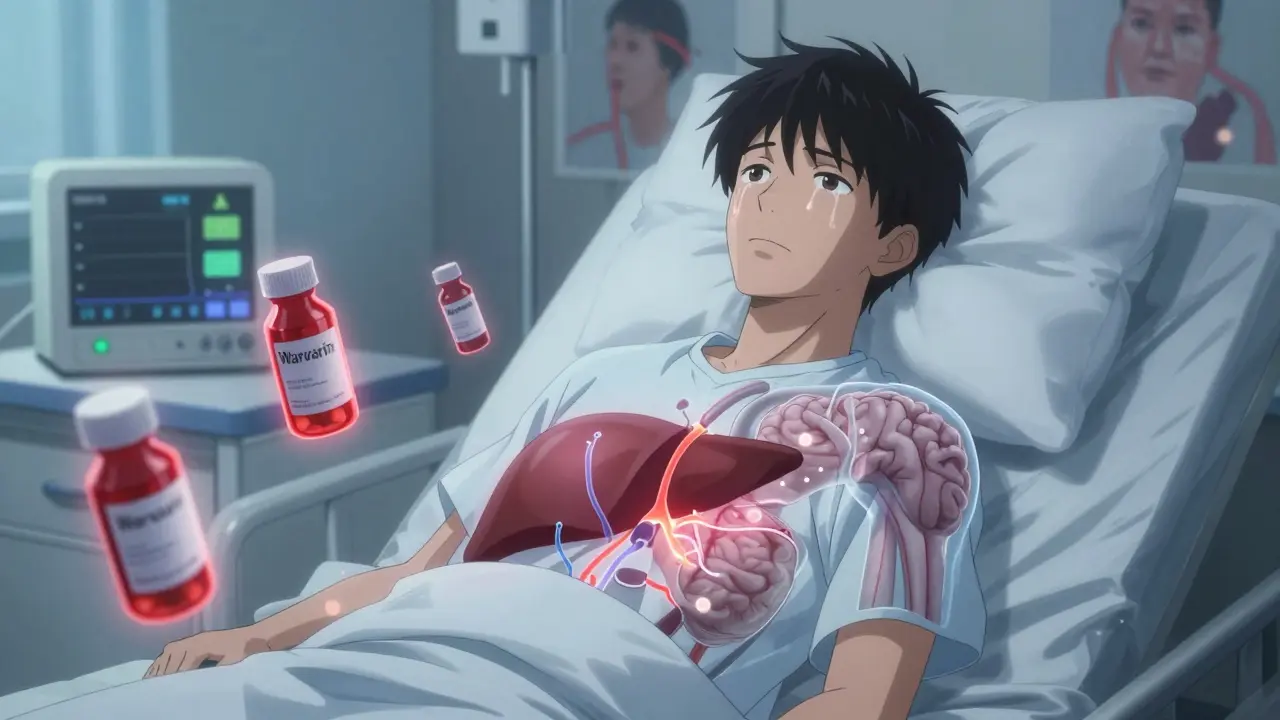
Drug Interactions You Can’t See Coming
Most drug interaction guides list known conflicts: “Don’t take this with grapefruit juice.” “Avoid NSAIDs with blood thinners.” Nothing prepares you for this:- A patient on warfarin gets an AAV gene therapy for hemophilia. Three months later, their INR spikes without any dose change.
- A child with spinal muscular atrophy receives nusinersen (Spinraza) while enrolled in a gene therapy trial. Their fever and fatigue worsen-not from the disease, but from an immune clash between the two treatments.
- An adult with Duchenne muscular dystrophy takes corticosteroids for muscle support. After gene therapy, their liver enzymes rise, and the steroids become toxic.
These aren’t rare case reports. They’re symptoms of a system that hasn’t caught up. There’s no database of gene therapy-drug interactions. No algorithm predicts risk. No clinician can look up “AAV9 + sertraline” and get a warning.
The 15-Year Shadow
The FDA now requires 15 years of follow-up for gene therapies that integrate into DNA or can remain latent-like those using herpesvirus or lentiviral vectors. Why 15 years? Because that’s how long it took for the leukemia cases to appear after SCID-X1 trials. That’s how long it took for immune tolerance to break down in some AAV patients, leading to sudden loss of gene expression and liver damage.But here’s the catch: most drug trials last two to five years. By the time a gene therapy gets approved, we still don’t know how it interacts with the medications patients will be taking in year 8, 10, or 12. A patient might be fine for five years, then start having seizures because their antiepileptic drug suddenly stopped working. Why? Because the gene therapy altered their brain’s inflammatory environment. No one tested that.
Who Gets Left Out?
Clinical trials for gene therapy are small. They often exclude people on multiple medications, elderly patients, or those with liver or kidney disease. That means the people who need these therapies most-those already on complex drug regimens-are the least studied.Imagine a 65-year-old with a genetic heart condition who’s also on beta-blockers, statins, and aspirin. They’re a perfect candidate for gene therapy. But they were excluded from the trial because they took more than three drugs. So when they finally get treatment, no one knows if the therapy will make their statin toxic. Or if their blood thinner will suddenly become ineffective. The risk is real. The data? Nonexistent.

What’s Being Done?
Regulators are trying. The FDA and EMA now require sponsors to test gene therapies alongside common drugs in preclinical studies. They ask for data on immune activation, liver enzyme changes, and vector shedding. But real-world data is still scarce.Some research centers are building registries. The UK’s National Gene Therapy Registry tracks patients long-term, noting every medication they take. Early results show that nearly 40% of gene therapy recipients require adjustments to their existing drugs within the first year. Most adjustments are minor. But a few are life-saving.
Scientists are also developing biomarkers-blood tests that predict immune overreaction before it happens. One promising marker measures levels of interferon-alpha and IL-6 after vector infusion. High levels? That patient is at risk for drug metabolism disruption. But these tests aren’t routine yet. And they don’t exist for every therapy.
What Patients and Doctors Need to Know
If you’re considering gene therapy:- Make sure your doctor knows every medication you take-prescription, over-the-counter, supplements, even herbal teas.
- Ask if the therapy has been tested with any of your drugs. If not, assume there’s a risk.
- Expect changes in how your medications work. You might need more frequent blood tests.
- Don’t stop or start any drug without consulting your gene therapy team.
- Know that side effects could show up years later. Keep records. Stay in touch with your care team.
For clinicians: Don’t treat gene therapy like a new drug. Treat it like a new biology. Your patient’s entire metabolic landscape has changed. Monitor more than just the disease. Monitor their drug levels. Watch for unexplained symptoms. Be ready to adjust. And never assume safety just because the therapy passed a clinical trial.
The Future Isn’t Just About Better Vectors
The next big leap won’t come from smarter viruses. It’ll come from better tracking. We need real-time monitoring of gene expression, immune response, and drug metabolism in real patients. We need global databases that link gene therapy use to long-term drug outcomes. We need guidelines that tell doctors: “If a patient on AAV8 gets this drug, check these three things.”Until then, every gene therapy patient walks a tightrope. They’re not just treating a disease. They’re stepping into an uncharted zone where their body’s rules have changed-and no one has a map.
Can gene therapy interact with over-the-counter drugs?
Yes. Even common supplements like St. John’s wort, fish oil, or vitamin E can interfere with gene therapy. These substances affect liver enzymes and immune responses, just like prescription drugs. Patients should disclose everything they take-even if they think it’s harmless.
How long should I wait after gene therapy before starting a new medication?
There’s no universal answer. For AAV therapies, many teams recommend waiting 3-6 months to let the immune system settle. For integrating vectors, waiting a year or more may be safer. But this depends on the therapy, the patient’s immune status, and the drug involved. Always consult your gene therapy team before starting anything new.
Are children at higher risk for drug interactions with gene therapy?
Children’s immune systems and drug metabolism pathways are still developing, making them more vulnerable to unpredictable interactions. A therapy that’s safe in adults may cause unexpected liver toxicity or immune overreaction in kids. That’s why pediatric gene therapy trials are so tightly monitored-and why long-term follow-up is critical.
Can gene therapy affect how birth control works?
Potentially. Hormonal contraceptives are metabolized by liver enzymes that can be altered by gene therapy-induced inflammation. There’s no direct data yet, but experts advise using non-hormonal contraception (like IUDs) during and after gene therapy until more is known. Always discuss this with your care team.
What if I need emergency surgery after gene therapy?
Tell every doctor involved-surgeons, anesthesiologists, nurses-that you’ve had gene therapy. Anesthesia drugs, antibiotics, and painkillers are metabolized by the liver and kidneys, which may be affected by the therapy. A standard dose could become toxic. Your gene therapy team should provide a medical alert card or summary to carry with you.
Jay Amparo
January 9, 2026 AT 21:18Man, I read this and just sat there for five minutes staring at my coffee. It’s wild how we’re basically playing God with biology and expecting everything to behave like a textbook. I’ve got a cousin on statins for life, and now they’re considering gene therapy for familial hypercholesterolemia. No one’s even asked if the therapy will turn his liver into a hostile zone for his meds. We’re flying blind here, and it’s terrifying.
But also? Kinda beautiful. We’re fixing diseases that used to mean a death sentence. Just wish the safety nets were woven tighter before we let people jump.
Lisa Cozad
January 10, 2026 AT 05:37This is exactly why I switched from clinical research to patient advocacy. I’ve sat in too many rooms where doctors say, ‘We don’t know yet,’ and then hand out a consent form like it’s a coupon. The fact that we’re not tracking every single medication a patient takes before, during, and after therapy is insane. Even OTC stuff like turmeric or melatonin can throw off liver enzymes. If you’re getting gene therapy, your whole pharmacology profile should be mapped like a satellite orbit.
And yes, I’m yelling at my screen right now.
Saumya Roy Chaudhuri
January 10, 2026 AT 21:56Oh please. This is just fearmongering dressed up as science. Gene therapy has been around for decades. The 1999 case? That was adenovirus - outdated tech. Modern AAV vectors are clean, targeted, and regulated like rocket science. The ‘unpredictable interactions’ are anecdotal noise. If your doctor can’t handle a few drug level fluctuations, maybe they shouldn’t be prescribing anything.
Also, St. John’s wort? Really? You’re comparing herbal tea to a precision gene edit? Get real. This article reads like a TED Talk written by someone who thinks CRISPR is a brand of energy drink.
Ian Cheung
January 11, 2026 AT 05:22Imagine your body’s a car and gene therapy is a custom turbocharger installed by a guy who’s never driven before
Now imagine your meds are the fuel and oil and brake fluid
And you’re told ‘it’s fine we tested it for 3 months’
But the turbo’s gonna keep spinning for 15 years
And the oil filter? It’s now made of feelings
And the mechanic who built it? They’re on vacation
And your mechanic from 2018? Still trying to fix the transmission you never asked them to touch
Yeah. We’re all just roadkill waiting for a signal
Also I just took ibuprofen and now I’m scared to breathe
anthony martinez
January 12, 2026 AT 17:20So let me get this straight. We’re inserting viral DNA into people’s cells, altering their biology permanently, and then acting surprised when their blood pressure meds stop working? And the FDA says ‘15 years of follow-up’ like that’s some kind of heroic effort?
Bro. We’re not running a clinical trial. We’re running a long-term experiment on humans with no exit strategy. And the worst part? The people who need this most are the ones we excluded from the study because they were ‘too complex.’
Genius.
Mario Bros
January 13, 2026 AT 18:16Hey - if you’re even thinking about gene therapy, DO NOT skip the pre-screening. I know it’s a pain. I’ve been there. But trust me, your doc needs to know EVERYTHING - even that tea you drink that says ‘natural immune booster’ on the box.
My buddy got the SMA therapy last year. Took his antidepressant like normal. Three weeks later, he was in the ER with liver enzymes through the roof. Turned out the therapy spiked his CYP3A4 like a volcano.
He’s fine now. But he almost wasn’t. Talk to your team. Keep records. Don’t be the guy who says ‘it’s just a supplement.’
❤️
Jake Nunez
January 15, 2026 AT 13:03As someone who grew up in a country where gene therapy is still a sci-fi concept, I find this both fascinating and terrifying. In India, we’re still struggling to get basic medications to rural clinics. Meanwhile, here you’re debating whether your blood thinner will suddenly become poison because of a viral vector.
It’s not just a medical issue - it’s a global equity issue. Who gets to be part of this ‘precision medicine’ future? The wealthy? The well-documented? The ones who can afford 15 years of follow-up visits?
Let’s not fix genes and forget people.
Christine Milne
January 15, 2026 AT 13:51This article is an egregious misrepresentation of regulatory science. The FDA does not permit gene therapies to proceed without exhaustive preclinical pharmacokinetic and immunogenicity profiling. The so-called ‘unknown interactions’ are not unknown - they are actively being characterized under IND protocols and post-marketing surveillance requirements. The notion that clinicians are ‘flying blind’ is demonstrably false and dangerously misleading.
Furthermore, the exclusion criteria in clinical trials are not arbitrary - they are scientifically necessary to isolate therapeutic effects. To imply that patients on polypharmacy are being discriminated against is to misunderstand the fundamental purpose of controlled trials.
Do not confuse anecdotal case reports with systemic failure.
Bradford Beardall
January 15, 2026 AT 21:51Wait - so if I get gene therapy for my inherited cardiomyopathy, and then 8 years later I need an antibiotic for pneumonia, I could end up with liver failure because my CYP enzymes got rewired? And there’s no database to check if azithromycin + AAV9 = bad news?
That’s not a medical system. That’s a Russian roulette game with a 15-year timer.
Who’s building this database? Is it NIH? Is it Pharma? Is it some grad student in a basement with a spreadsheet? Because if it’s the latter, we’re all screwed.
Also - does anyone know if this affects IVF? Just wondering.
McCarthy Halverson
January 16, 2026 AT 23:40Know your meds. Tell your team. Get blood tests. Don’t guess. Stay in touch. That’s it.
Simple. But nobody does it.
And that’s why people get hurt.
Dwayne Dickson
January 17, 2026 AT 12:53From a translational medicine standpoint, the paradigm shift here is not merely pharmacokinetic - it is ontological. The introduction of a stably expressed transgene into a somatic cell population constitutes a novel biological entity with emergent metabolic properties that cannot be predicted by classical pharmacodynamic models.
The regulatory framework, as currently constituted, is predicated on the assumption of transient pharmacologic exposure. Gene therapy, by its very nature, obviates this assumption. Consequently, the traditional drug interaction lexicon is rendered semantically obsolete.
What we require is not merely a database, but a dynamic, longitudinal, multi-omic phenotyping architecture capable of real-time integration of vector biodistribution, immune modulation, and metabolomic flux - all correlated against concomitant pharmaceutical exposure.
And no - your doctor’s EHR is not going to cut it.
But I’m glad you’re talking about it. Progress, albeit glacial, is occurring.